AstraZeneca withdraws COVID vaccines after millions took their jabs
After millions of people took the AstraZeneca vaccine as their choice of vaccination during the COVID-19 pandemic, the organization announced on March 5, that it is withdrawing its vaccines from the market worldwide, effective as of May 7 as it was reported to cause side effects.
It is said 170 countries received doses of AstraZeneca; it was expected 35.3 million doses would be shared with 36 Caribbean and Latin American countries through the COVAX program, according to PAHO.
American countries through the COVAX program, according to PAHO.
First reported by the Telegraph, the side effects are identified as blood clots and low blood platelet counts. In fact, the medical issues have reportedly been linked to 81 confirmed deaths in the UK alone. Many others are suffering some other medical injury due to the vaccine popularized as an answer to the then new virus.
AstraZeneca is also being sued by more than 45 affected people.
The vaccine, known as Vaxzevria, the Telegraph informs, can no longer be used in the European Union and it will be the same for other countries and the UK in the coming months. Over 17 million doses made it into EU countries.
Reports say AstraZeneca made the revelations of the side effects in court documents, adding that the withdrawal of their vaccine is also due to a decrease in demand.
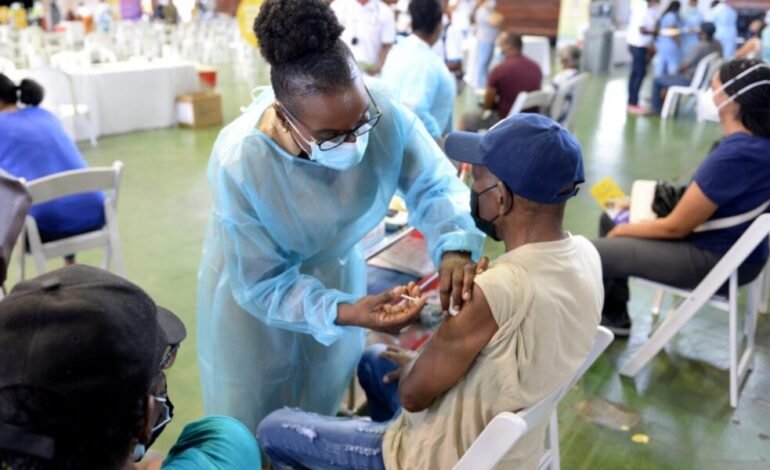
Photo Caption: Public Health Nurse, Patricia Coates (left), administers Daniel Adams’ first dose of the AstraZeneca coronavirus (COVID-19) vaccine, during the second COVID-19 Vaccination Blitz at the National Arena in St. Andrew, on Saturday (April 3).







